Is it diversity or just a mess? When RNA splicing does not go according to plan
Shaked Shanas# and Martin Mikl - Department of Human Biology
Judith Somekh - Department of Information Systems
Machine Learning
Deep learning
Neural Networks
Bioinformatics
PhD Grant 2023
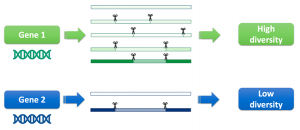 The genes in our bodies create the proteins we need to live. Some genes can create multiple proteins, which is possible through alternative ways how the genetic information can be processed. The main molecular mechanism to create a diverse range of proteins from the same gene is splicing. During the process of splicing, parts of the genetic information are cut out from the transcribed RNA and the flanking sequences are combined together to give the final RNA which is the template for protein synthesis. A single gene can give rise to multiple proteins by generating different RNAs through alternative processing by the splicing machinery – a mechanism called alternative splicing. The proteins generated through alternative splicing can be beneficial, or they can constitute potentially harmful products of errors in splicing.
The genes in our bodies create the proteins we need to live. Some genes can create multiple proteins, which is possible through alternative ways how the genetic information can be processed. The main molecular mechanism to create a diverse range of proteins from the same gene is splicing. During the process of splicing, parts of the genetic information are cut out from the transcribed RNA and the flanking sequences are combined together to give the final RNA which is the template for protein synthesis. A single gene can give rise to multiple proteins by generating different RNAs through alternative processing by the splicing machinery – a mechanism called alternative splicing. The proteins generated through alternative splicing can be beneficial, or they can constitute potentially harmful products of errors in splicing.
Expression of our genes is tightly regulated to create the most suitable proteins for any situation and for any cell type. Many diseases are associated with defects in splicing, demonstrating the importance of correct processing of the genetic information. However, the regulation and functional importance of the diversity of spliced gene products is largely unknown.
In a collaboration of the Mikl and Somekh labs, we aim to describe and analyze the diversity of splice outcomes and explore how environmental or cellular factors affect this diversity.
First of all, we observed that genes can produce many more splice outcomes than we know about today. Unknown splice isoforms are often referred to as “noise” or “junk”. One of our goals is to determine if this “noise” might tell a story – are these simply mistakes or a well-planned evolutionary strategy?
In addition, we found that genes can have different diversity of splicing outcomes depending on the tissue type. This shows that the splicing diversity is characteristic of a cell with a specific function. In the future, this insight could lead us to identify which cellular factors are involved in creating this diversity, whether we should call them “mistakes” or “smart adaptation strategies” and how we could use this information to correct mis-splicing in disease.

The genes in our bodies create the proteins we need to live. Some genes can create multiple proteins, which is possible through alternative ways how the genetic information can be processed. The main molecular mechanism to create a diverse range of proteins from the same gene is splicing. During the process of splicing, parts of the genetic information are cut out from the transcribed RNA and the flanking sequences are combined together to give the final RNA which is the template for protein synthesis. A single gene can give rise to multiple proteins by generating different RNAs through alternative processing by the splicing machinery – a mechanism called alternative splicing. The proteins generated through alternative splicing can be beneficial, or they can constitute potentially harmful products of errors in splicing.
Expression of our genes is tightly regulated to create the most suitable proteins for any situation and for any cell type. Many diseases are associated with defects in splicing, demonstrating the importance of correct processing of the genetic information. However, the regulation and functional importance of the diversity of spliced gene products is largely unknown.
In a collaboration of the Mikl and Somekh labs, we aim to describe and analyze the diversity of splice outcomes and explore how environmental or cellular factors affect this diversity.
First of all, we observed that genes can produce many more splice outcomes than we know about today. Unknown splice isoforms are often referred to as “noise” or “junk”. One of our goals is to determine if this “noise” might tell a story – are these simply mistakes or a well-planned evolutionary strategy?
In addition, we found that genes can have different diversity of splicing outcomes depending on the tissue type. This shows that the splicing diversity is characteristic of a cell with a specific function. In the future, this insight could lead us to identify which cellular factors are involved in creating this diversity, whether we should call them “mistakes” or “smart adaptation strategies” and how we could use this information to correct mis-splicing in disease.

